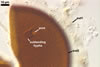
Layer
1 flexible, hyaline, (0.5-)1.0(-1.5) µm thick, staining
yellowish white (4A2) to pastel yellow (3A4) in Melzer’s reagent.
This layer expands in PVLG into a halo with radiate columns that disappear
with time. When present, this structure resembles a corona. The distance
between the outer surface of the expanded layer 1 and that of the laminate
layer 2 is up to 40 µm.
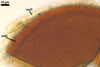
Layer
2 laminate, pale yellow (3A3) to dark yellow (4C8), (2.0-)8.5(-16.0)
µm thick, ornamented with evenly distributed hemispherical warts,
0.6-1.0 µm wide at their base and 0.5-1.0 µm high; layer 2 stains
burnt Sienna (7D8) to English red (8D8) in Melzer’s reagent.
Layer
3 flexible to semirigid, hyaline to pale yellow (4A3), ca.
0.8-2.2 µm thick, usually easily separating from layer 2 in crushed
spores, nonreactive in Melzer's reagent.
GERMINATION.
A
germ tube emerges from the lumen of the subtending hypha.
MYCORRHIZAE.
Many attempts to establish one-species cultures of Gl. pansihalos
with different plant hosts failed. No literature data exists of the properties
of mycorrhizae of this fungus.
DISTRIBUTION.
In Poland, Gl. pansihalos has been found to occur in different dune
sites adjacent to the Baltic Sea (Błaszkowski 1993; Tadych and Błaszkowski
2000) and in a calamine spoil mound located near Krakow (50o3'N, 19o57'E;
Pawlowska et al. 1996).
Glomus pansihalos
probably occurs in the whole world. It has been originally described based
on spores found in dune soils of California, New Jersey, and Michigan, and
from forest soils of southern Ontario, Canada (Berch and Koske 1986). Later,
Koske (1987) isolated this fungus from dunes extending from New Jersey to
Virginia. Halvorson and Koske (1987) and Koske and Halvorson (1989) recorded
Gl. pansihalos in dunes of San miguel island.
NOTES.
When observed under a dissecting microscope, spores of Gl. pansihalos
most resemble those of Gl. constrictum
Trappe. They are similar in colour and size. Additionally, the thick and colourless
outer spore wall layer of the latter species also produces a halo that is
reminiscent of the expanding wall layer of spores of the former fungus.
Examination of crushed
spores under a light microscope readily separates the two species. The outer
spore wall layer of Gl. constrictum is rigid in young and mature
spores and does not swell in lactic acid-based mountants. Additionally, the
surface of the laminate wall layer of spores of Gl. constrictum is
smooth (vs. warty in Gl. pansihalos) and the spore wall lacks the
third layer of spores of Gl. pansihalos.
REFERENCES
Berch S. M., Koske R.
E. 1986. Glomus pansihalos: a new species in the Endogonaceae, Zygomycetes.
Mycologia 78, 838-842.
Koske R. E. 1987. Distribution
of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia
79, 55-68.
Koske R. E., Halvorson
W. L. 1989. Mycorrhizal associations of selected plant species from San Miguel
Island, Channel Islands National Park, California. Pacific Sci. 43, 32-40.
Halvorson W. L., Koske
R. E. 1987. Mycorrhizae associated with an invasion of Erechtites glomerata
(Asteraceae) on San Miguel Island, California. Madrono 34, 260-268.
Pawlowska
T. E., Błaszkowski J., Rühling Å. 1996. The mycorrhizal status
of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza
6, 499-505.
Tadych M., Błaszkowski
J. 2000. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.